Gene-editing tool shown to limit impact of certain parasitic diseases
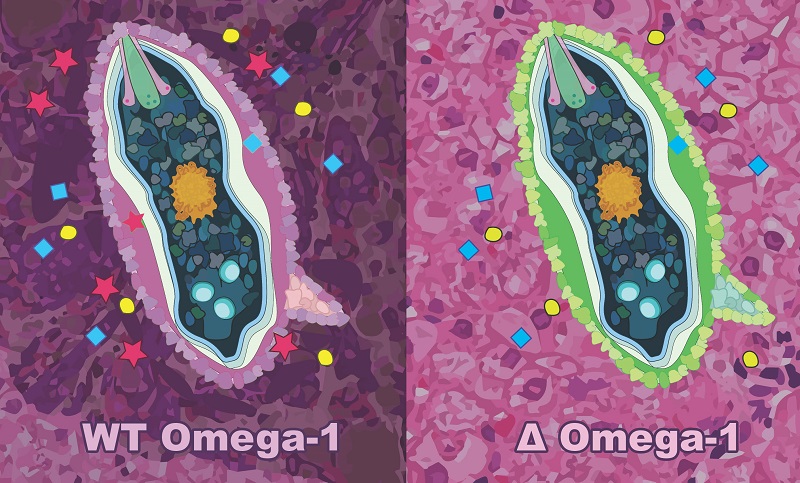
Using CRISPR/Cas9 to ‘knock-out’ omega-1 from schistosome eggs. The left panel represents a wild-type schistosome egg secreting pathogenic proteins (including omega-1; red stars) into host tissues. Eggs subjected to CRISPR/Cas9 genome editing in this study do not secrete omega-1 (right panel) into host tissues and are not pathogenic to the host.
16 January 2019
A parasitologist at Aberystwyth University is one of a worldwide team of researchers that has used a gene-editing tool to limit the impact of schistosomiasis, an illness that affects more than a quarter of a billion people in Southeast Asia, sub-Saharan Africa, and Latin America.
Professor Karl Hoffmann from the Institute of Biological, Environmental and Rural Sciences (IBERS) has been working with researchers at the George Washington University (GW) and colleagues at institutes in Thailand, Australia, the UK, the Netherlands, and others.
For the first time CRISPR/Cas9 has been successfully used to limit the impact of parasitic worms responsible for schistosomiasis and for liver fluke infection, which can cause a diverse spectrum of human disease including liver fibrosis and bile duct cancer.
Their findings are found in two papers published today in the journal eLife. Professor Hoffmann is co-author of the paper entitled “Programmed genome editing of the omega-1 ribonuclease of the blood fluke, Schistosoma mansoni”.
Professor Hoffmann’s work at Aberystwyth University focuses on tackling schistosomiasis.
Professor Hoffmann said: “With funding provided by the Wellcome Trust and working with colleagues at GW, we have been able to apply, for the first time in flukes, genome editing to knock-out an important schistosome gene product involved in human disease. Application of this technology to other schistosome genes or flukes will revolutionise our ability to develop new and urgently-needed control strategies.”
“The genes we ‘knocked out’ using CRISPR/Cas9 resulted in markedly diminished symptoms of infection in our animal models,” said Paul Brindley, PhD, professor of microbiology, immunology, and tropical medicine at the GW School of Medicine and Health Sciences, and lead author.
“Our research also showed that this revolutionary new biomedical procedure — CRISPR/Cas9 — can be adapted to study helminth parasites, which are a major public health problem in tropical climates.”
CRISPR/Cas9 is a new technology that allows researchers to precisely target and deactivate the genetic information needed to produce a particular protein. While the tool has been used in other species before, it was unknown if it could be applied to Schistosoma mansoni and Opisthorchis viverrini, the parasites responsible for schistosomiasis and liver fluke infection.
Schistosomiasis can cause serious health problems, including damage to the liver and kidneys, infertility and bladder cancer. The freshwater worms S. mansoni enter the human body by burrowing into the skin; once in the bloodstream, they move to various organs where they rapidly start to reproduce and produce pathogenic eggs.
These eggs release several molecules, including a protein known as omega-1 ribonuclease, which can damage the surrounding tissues. Brindley and his research team “knocked out” this protein using CRISPR/Cas9 and found that it greatly reduced the impact of the disease.
“This neglected tropical disease affects more than a quarter of a billion people primarily living in Southeast Asia, sub-Saharan Africa, and Latin America,” said Brindley. “CRISPR/Cas9 is a tool that may be used to limit the impact of these infections. As we work to better understand how these parasites invade and damage our bodies through this new technology, we will find new ideas for treatment and disease control.”
Key collaborators include Karl Hoffmann, PhD at the Aberystwyth University; Thewarach Laha, PhD at Khon Kaen University, Thailand; and Alex Loukas, PhD at James Cook University, Australia.
The papers, entitled “Programmed genome editing of the omega-1 ribonuclease of the blood fluke, Schistosoma mansoni” and“Programmed knockout mutation of liver fluke granulin attenuates virulence of infection-induced hepatobiliary morbidity,” are available at eLife.
Ends.